Rutherford along with his colleagues did a famous gold foil experiment to determine the atom is not like a tennis ball or billiard ball, but it does have a central dense region which we call the nucleus and the electrons revolve along the nucleus.
The started with a very tin gold foil. They took the piece of the gold foil and shot tiny little particles at them and these particles were nothing but alpha particles.
What the wanted to do was that they wanted to hit the gold foil with alpha particles (α) and detect what happens once the alpha particles hit the particles in the gold foil. To detect that they had a material which would flash if the alpha particle would hit it. So they took the material, bend it into a circle a circle and put the gold foil right into its center of the material.
Now they shoot the alpha particles (α) from the side of the gold foil when there is an opening. Most of the time they saw flashes right behind the gold foil.
This indicates that the alpha particles (α) go right through the gold foil landing up on the other side, sometimes exactly behind the gold foil and in some cases, much to there surprise at the sides.This meant that the alpha particles were hitting the foil and deflecting off something or bouncing off something.
So, we can summarize what they saw with a diagram like this.

What the wanted to do was that they wanted to hit the gold foil with alpha particles (α) and detect what happens once the alpha particles hit the particles in the gold foil. To detect that they had a material which would flash if the alpha particle would hit it. So they took the material, bend it into a circle a circle and put the gold foil right into its center of the material.
Now they shoot the alpha particles (α) from the side of the gold foil when there is an opening. Most of the time they saw flashes right behind the gold foil.
This indicates that the alpha particles (α) go right through the gold foil landing up on the other side, sometimes exactly behind the gold foil and in some cases, much to there surprise at the sides.This meant that the alpha particles were hitting the foil and deflecting off something or bouncing off something.
So, we can summarize what they saw with a diagram like this.
So, here is the circular detector screen and there is the alpha particle (α) emitter, something like the gun shoots out the alpha particle (α) and the gold foil is in the center. And most of the time when the alpha particles would go right through and they would hit here and sometimes they would deflect off, like you can actually over here. In fact, some of the rays would actually bonce right back.
Now, lets look at the very important conclusions that they came to.
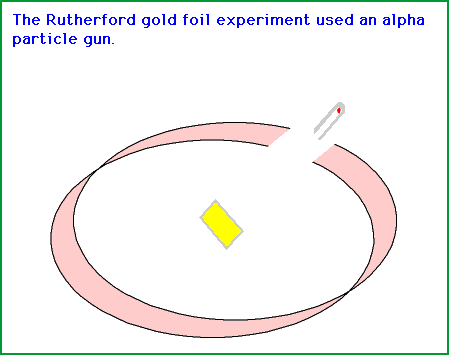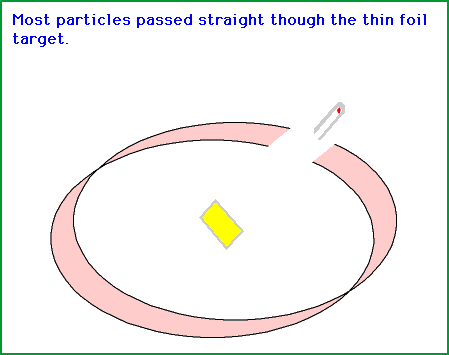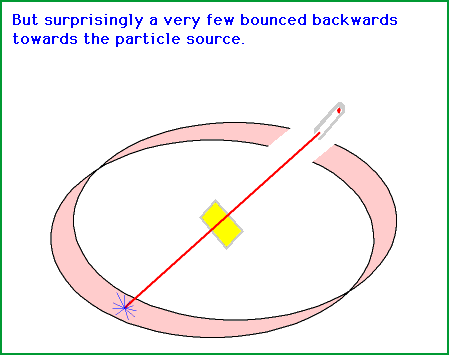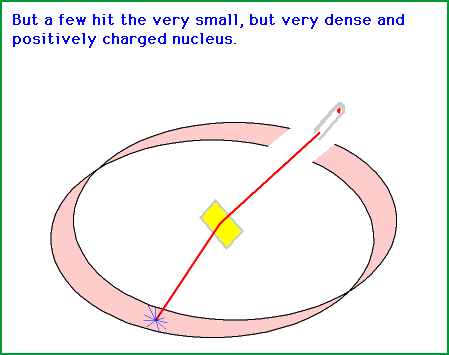
They reasoned that most of the atom would be empty space or something so that for light it was very easy to go right through them. Its not very dense and very easy to alpha particle to shoot through. The second thing they see is that once in a while the alpha particle actually hits off and goes on to the side and they reasoned that it should be because of nucleus but at that time they knew this.
The found it withe help that they knew that the alpha particle was positive in nature so the reason of being deflected was that their center would also be positive.
So, the conclusion was that there was something very hard and dense in the atom and once the alpha particle was hitting the hard and dense part it gets deflected.
So the conclusion of the experiment is that, when alpha particles are sot towards the gold foil most of the alpha particles pass through because they are not hitting the dense material in the atom. And, if the alpha particle hits the dense material they get repelled by the charge or the get bounced back.
And this is how the gold foil experiment changed the way the scientists think about the nucleus.
Comments
Post a Comment